AC Immune’s execution strategy mirrors our precision medicine approach: powered by our proprietary discovery platforms, SupraAntigen® and Morphomer®, we are establishing a unique portfolio of therapeutic and diagnostic programs capable of addressing the heterogeneity of Alzheimer’s disease and other neurodegenerative diseases.
AC Immune's Execution Strategy
Our strategy builds on three pillars:
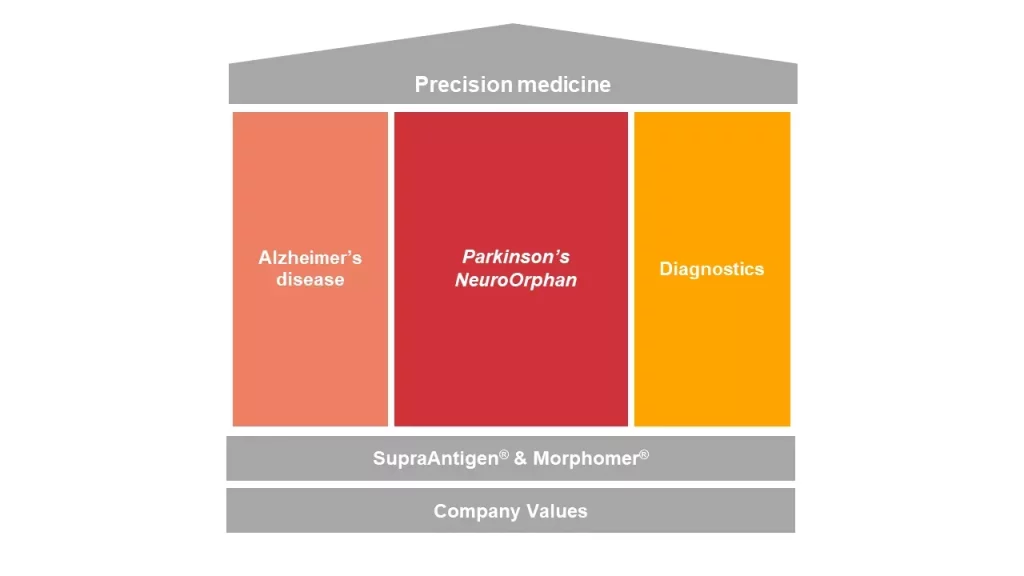

In partnership or independently, from clinical development to potential commercialization, we are accelerating late-stage biomarker-based development of our therapeutics for Alzheimer’s disease and spearheading vaccination for neurodegenerative diseases.
AC Immune is advancing novel therapeutic and diagnostic candidates targeting alpha-synuclein (a-syn), as the primary focus in Parkinson’s disease. Our industry-leading Parkinson’s vaccine candidate and our first-in-class a-syn PET tracer demonstrate the uniqueness and progress of AC Immune’s a-syn targeting programs.
In parallel, our pipeline also now includes NeuroOrphan indications caused by Tau- and TDP-43-driven diseases, the hallmarks of frontotemporal lobar degeneration (FTLD) and amyotrophic lateral sclerosis (ALS).


Up to 30% of people meeting currently used clinical criteria for the diagnosis of Alzheimer’s disease may be misdiagnosed and receive the wrong treatment.
In this context, diagnostic products will have a key role in creating a new treatment paradigm. In addition, earlier more reliable diagnosis based on biomarkers will allow treatment at early stages of disease and may ultimately enable preservation of function in mild or even preclinical stages.
THERAPEUTIC FOCUS
Alzheimer’s disease is the most prevalent neurodegenerative disease leading to dementia and a major focus of AC Immune. In recent years, as our pipeline has grown, we have diversified to develop product-candidates targeting Parkinson’s disease and other NeuroOrphan diseases.


Morphomer and SupraAntigen
Discover AC Immune’s technology platforms that generated our differentiated pipeline of diagnostic and therapeutic candidates.